A phase 3 randomized, double-blind, 52-week placebocontrolled multi-center study with a double-blind 52-week extension period with randomized dose up/dose down titration investigating the efficacy, safety, and tolerability of Ritlecitinib in adult participants with nonsegmental vitiligo. Register Today!
Vaccine (RSV) – 222090 (extension to 212494)
A phase 3b, randomized, open label, multi-country, multi-center, extension and crossover vaccination study to evaluate the immunogenicity and safety of different revaccination schedules and persistence of a single dose of the RSVPreF3 OA vaccine in adults aged 60 years and above who participated in the RSV OA=ADJ-006 study. Register Today!
Atopic Dermatitis – M16-047 (extension to M18-891)
A Phase 3 Randomized, Placebo-Controlled, Double-Blind Study to Evaluate Upadacitinib in Combination with Topical Corticosteroids in Adolescent and Adult Subjects with Moderate to Severe Atopic Dermatitis For more information, please click here. Register Today!
Crohn’s Disease (M14-430)
Crohn’s disease is an inflammatory bowel disease (IBD). It causes inflammation of the lining of your digestive tract, which can lead to abdominal pain, severe diarrhea, fatigue, weight loss and malnutrition. Inflammation caused by Crohn’s disease can involve different areas of the digestive tract in different people. The inflammation caused by Crohn’s disease often spreads…
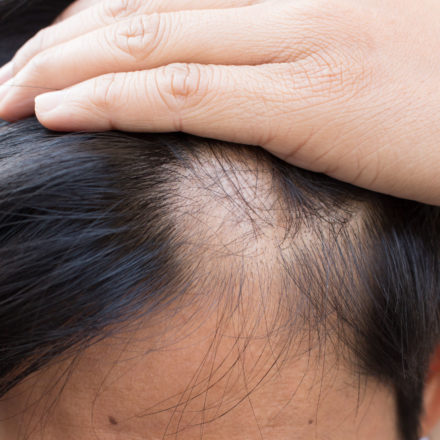
Alopecia Areata – M23-716
A Phase 3 randomized, placebo-controlled, double-blind program to evaluate efficacy and safety of upadacitinib in adult and adolescent subjects with severe alopecia areata Register Today!
Ulcerative Colitis (M14-533)
M14-533 is an extension study of M14-234. The purpose of this study is to evaluate the long-term safety and efficacy of ABT-494 in subjects with Ulcerative Colitis (UC) who have not responded at the end of the induction period in Study M14-234, who have been inadequate responders during the maintenance period of Study M14-234, or…